-
-
-
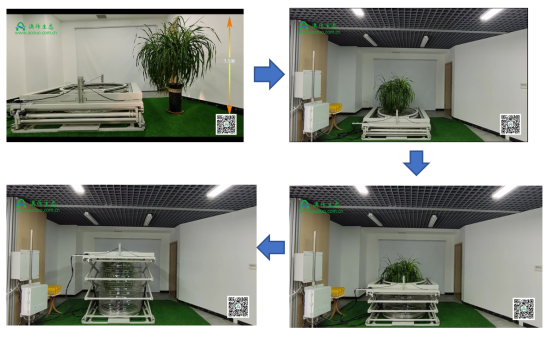
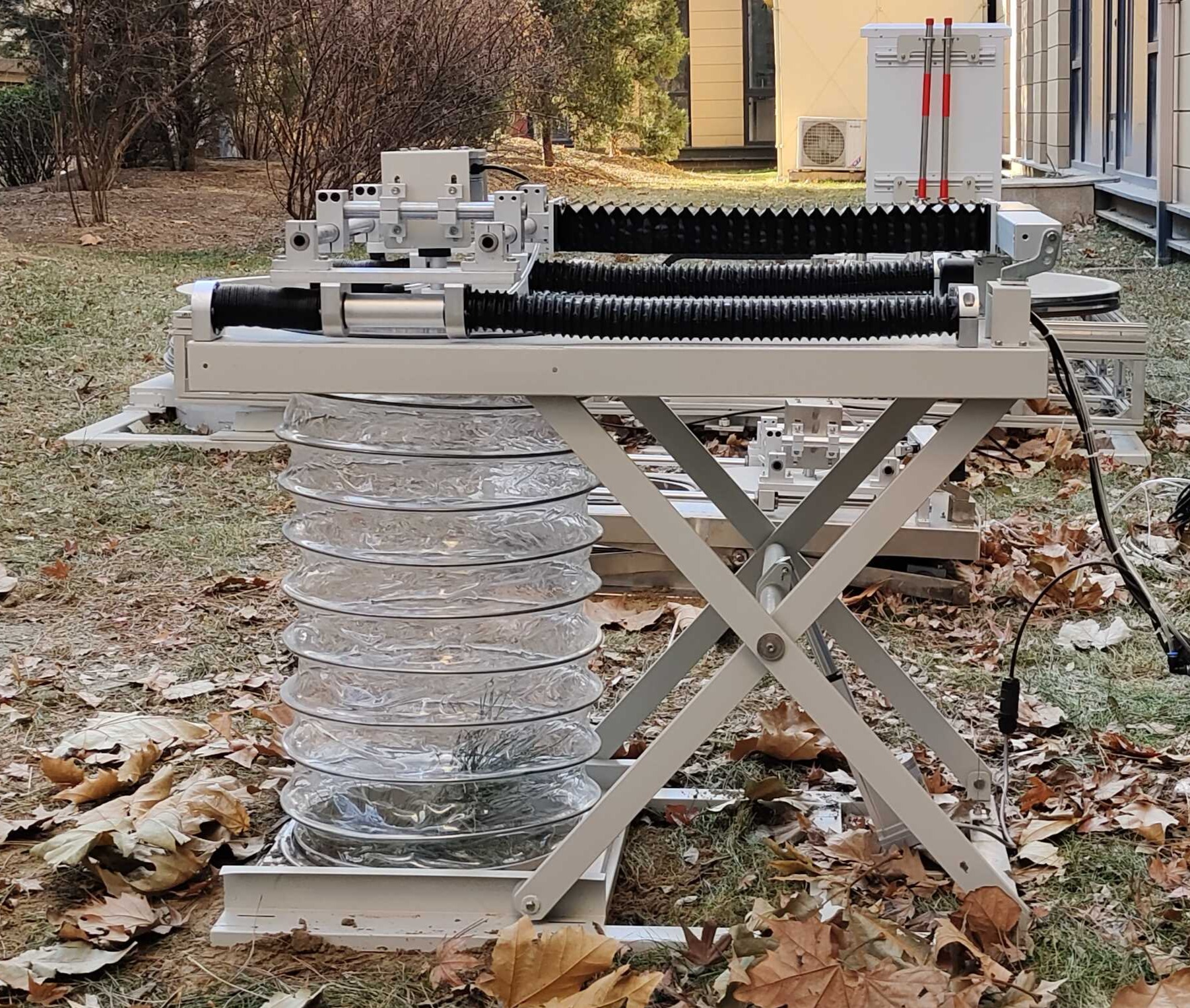
SoilScope 控制型蒸渗实验系统(称重式地中蒸渗仪)
넶1199 ¥ 0.00 -
LysiCosm 碳氮水耦合过程监测系统
넶678 ¥ 0.00 -
SmartSoil 野外增温试验系统
넶421 ¥ 0.00 -
ENVIdata-ET 原位蒸散网络化监测系统
넶486 ¥ 0.00
-
-
-
soilgas便携式温室气体监测仪
넶175 ¥ 0.00 -
iChamber群落全株自动箱
넶358 ¥ 0.00 -
CPEC-AZ升级涡度通量及土壤通量同步观测系统
넶514 ¥ 0.00 -
EcoChem激光光谱元素分析系统
넶1438 ¥ 0.00
-
-
-
AZR-300复合根系生长动态监测系统
넶1684 ¥ 0.00 -
Rhizoscope原位根系3D观测系统
넶605 ¥ 0.00 -
RhizoCam 原位自动根系监测系统
넶830 ¥ 0.00 -
AZR-300TF复合根系荧光监测系统
넶591 ¥ 0.00
-
-
-
EcoChem 种质资源检测鉴定系统
넶35 ¥ 0.00 -
EcoChem 中药材元素富集分析系统
넶38 ¥ 0.00 -
EcoChem 中药材溯源分析系统
넶32 ¥ 0.00 -
EcoChem 土壤质量监测与评价观测系统
넶35 ¥ 0.00
-
-
-
iChamber群落全株自动箱
넶358 ¥ 0.00 -
IRRIScope 灌溉指导器
넶318 ¥ 0.00 -
SeedScope 数字化育种控制实验系统
넶277 ¥ 0.00 -
AIM-WiFi土壤多参数监测系统
넶969 ¥ 0.00
-
-
-
AZG-300便携式土壤水体温室气体监测仪
넶1430 ¥ 0.00 -
EcoCS 生态碳汇能力监测
넶690 ¥ 0.00 -
iChamber-60 群落光合呼吸测量系统
넶371 ¥ 0.00
-
-
-
SONO-M1M2便携式水分速测仪
넶399 ¥ 0.00 -
SONO-WZ混凝土水分含量/水胶比测量仪
넶270 ¥ 0.00 -
SONO混凝土在线监测水分传感器
넶239 ¥ 0.00 -
SONO-Ex谷物水分测量系统
넶240 ¥ 0.00
-
-
-
-
- 2025-03-21
- 2024-10-11
- 2024-06-28
- 2024-06-18
- 2024-06-12
- 2024-03-26
- 2024-01-10
- 2024-01-04
-
- 2025-03-21
- 2025-03-21
- 2025-02-28
- 2025-01-24
- 2025-01-03
- 2025-01-03
- 2025-01-03
- 2025-01-03
-
- 2025-03-14
- 2024-11-22
- 2023-11-01
- 2023-08-03
- 2023-07-27
- 2023-02-15
-
- 2025-03-28
- 2025-03-14
- 2025-03-14
- 2025-03-14
- 2024-12-13
- 2024-12-13
- 2024-12-06
- 2024-11-29
-
- 2024-09-27
- 2024-09-27
- 2024-09-27
- 2024-08-22
- 2024-08-22
- 2024-01-04
- 2023-12-21
- 2023-12-07
-
- 2025-03-28
- 2025-03-07
- 2023-04-04
- 2023-04-04
-
- 2023-02-16
-
-
-
作为中国第一个以“生态仪器”命名的专业仪器公司,从成立之初,澳作生态仪器有限公司就致力于引进、推广国际先进的生态环境监测技术和仪器设备,并根据国内的科研需求研发、定制生态系统监测设施和仪器。时至今日,已经走过二十年的历程。
公司具有一支由实力雄厚的科研技术人员组成的团队,85% 以上具有本科或本科以上学历,其中一半人员具备硕士以上学历。公司总部位于中关村翠湖科技园云中心,在广州,南京、成都、郑州、泰安、新疆设立了营销、技术服务中心,网络化办公最大程度上给予客户周到便利的咨讯和服务。
产品
PSK植物胁迫测量套件
PSK植物胁迫测量套件
应用
Y(II)或ΔF/Fm’ 或 (Fm’ – Fs )/Fm’) 是经受时间考验的光适应测量参数,比Fv/Fm对更多类型的植物胁迫更加敏感。已有的大量证据表明Fv/Fm对许多种植物胁迫和健康植物的光系统II的测量十分出色,而Y(II)或光量子产额则可测量实际光照下光适应环境和生理状况的光系统II的效率。
 |
 |
| Y(II)测量仪 | Fv/Fm测量仪 |
原理
采用调制饱和脉冲原理,测量植物的叶绿素荧光,测量参数包括植物的光量子产额Y(II)及相对电子传递速率ETR,最大光化学效率Fv/Fm,同时还可测量PAR、叶温、相对湿度和叶片吸光率等环境参数。
特点
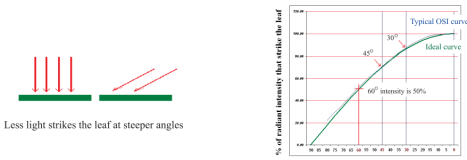
叶片吸光率测量:提供叶片吸收测量及随环境变化导致的叶片吸收变化。根据Eichelman (2004) 叶片吸收在健康植物的变化范围在0.7~0.9 之间。因此,为获得准确的ETR或“J”,Y(II)测量仪提供了一个可靠的测量方法,
Fv/Fm测量单元:用于暗适应测量。
 |
具有暗适应叶夹 阳光下屏幕可见 图形显示Fv/Fm曲线 2GB存储空间 USB通讯 数据Excel查看 |
 |
 |
Fm’校正:对于具有高光照强度历史的植物,完全关闭光反应中心是一个问题,Y(II)测量仪使用Loriaux &Genty 2013的方法进行Fm’ 校正,确保可以测得准确的Fm’ 。
自动调制光设定:快速准确自动的调整合适的调制光强,避免人工操作的误差。
先进算法避免饱和脉冲NPQ:采用25ms内8点的平均值确定Fm、Fm’、Fo、Fs,消除饱和脉冲NPQ的影响和电子噪音。
更精确的叶温测量:采用非接触式红外测量,测量精度可达±0.5℃。
直接测量相对湿度:含有测量气体交换使用的固态传感器,可测量相对湿度。
降低叶片遮挡的设计:倾斜的角度减少对叶片的遮挡,可以测量拟南芥等小叶。
系统组成
 |
标配: Y(II)光量子产额测量仪,Fv/Fm测量仪及10个暗适应叶夹,2个电池,2个充电器,一个便携箱,文件U盘。 |
技术指标
测量参数:
Y(II)或ΔF/Fm‘、ETR、PAR、Tleaf、相对湿度、Fms或Fm’、Fs、α(叶片吸收率)、FV/FM、FV/FO,FO, FM, FV。
监测模式:允许长时间监测
技术参数:
Y(II): 光适应测量, 稳态光合作用下的环境光
光源
饱和脉冲: LED白光源,使用PAR叶夹时可达7000μmols
调制光:红光,LED 660nm,具有690nm窄通过滤器。
光化光源:环境光
检测方法:脉冲调制法
PAR:测量400-700nm,余弦校正 ±2umols
Fv/Fm:暗适应测量
光源:LED红光饱和光闪,可达6000umols;
调制光:660nmLED 红光,690nm滤波器
调制光可以根据实际测量自动调节到合适的强度,减少手动调节误差,
相对湿度:0%~100%,±2%。
检测器&过滤器:具有700~750nm带通过滤的PIN光电二极管
可选配三脚架。
显示:132 X 30 pixel 液晶显示屏
取样速率:1~10000点/秒自动切换。
测量时间:最短3s或也可设置长期监测模式
存储空间:2GB
输出:USB下载数据,用Excel查看,无需安装其他专用软件
供电:USB锂离子电池(普通充电宝),可用8小时
尺寸:便携箱尺寸为14”x 11”x 6”,仪器为9’’长
质量:Y(II) 测量仪0.45 kg
Fv/Fm测量仪0.36 kg.
加便携箱和附件总重1.95 kg.
工作温度:0℃ ~ 50℃
产地
美国
文献
Adams & Demming-Adams 2004 – Chlorophyll Fluorescence as a tool to Monitor Plant Response to the Environment. William W. Adams III and Barbara Demmig-Adams, From Chapter 22, “Chlorophyll a Fluorescence a Signature of Photosynthesis”, edited by George Papaqeorgiou and Govindjee, published by Springer 2004, PO Box 17, 3300 AA Dordrecht, The Netherlands, pages 598 -599
Adams WW III, Demmig-Adams B. (1994) Carotenoid composition and down regulation of Photosystem II in three conifer species during the winter. Physiol Plant 92: 451-458
Ball MC. (1994) The role of photoinhibition during seedling establishment at low temperatures. In: Baker NR. And Bowyer JR. (eds) Photoinhibition of Photosynthesis. From Molecular Mechanisms to the Field, pp365-3376 Bios Scientific Publishers, Oxford
Ball MC., Butterworth JA., Roden JS., Christian R., Egerton JJG., (1995) Applications of chlorophyll fluorescence to forest ecology. Aust. J. Plant Physiology 22: 311-319
Baker N.R, Rosenquist E. (2004) Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities, Bukhov & Carpentier 2004 – Effects of Water Stress on the Photosynthetic Efficiency of Plants, Bukhov NG., & Robert Carpentier, From Chapter 24, “Chlorophyll a Fluorescence a Signature of Photosynthesis”, edited by George
Papaqeorgiou and Govindjee, published by Springer 2004, PO Box 17, 3300 AA Dordrecht, The Netherlands, page 627-628 Burke J. (2007) Evaluation of Source Leaf Responses to Water-Deficit Stresses in Cotton Using a Novel Stress Bioassay, Plant Physiology, Jan. 2007, Vol 143, pp108-121
Burke J., Franks C.D. Burow G., Xin Z. (2010) Selection system for the Stay-Green Drought Tolerance Trait in Sorghum Germplasm, Agronomy Journal 102:1118-1122 May 2010
Cavender-Bares J. & Fakhri A. Bazzaz 2004 – “From Leaves to Ecosystem: Using Chlorophyll Fluorescence to Assess Photosynthesis and Plant Function in Ecological Studies”. Jeannine Cavender Bares, Fakhri A. Bazzaz, From Chapter 29, “Chlorophyll a Fluorescence a Signature of Photosynthesis”, edited by George Papaqeorgiou and Govindjee, published by Springer 2004, PO Box 17, 3300 AA Dordrecht, The Netherlands, page 746-747 ETR Drought stress and npq
Cazzaniga S, Osto L.D., Kong S-G., Wada M., Bassi R., (2013) “Interaction between avoidance of photon absorption, excess energy dissipation and zeaxanthin synthesis against photo oxidative stress in Arabidopsis”, The Plant Journal, Volume 76, Issue 4, pages568–579, November 2013 DOI: 10.1111/tpj.12314
Cheng L., Fuchigami L., Breen P., (2001) “The relationship between photosystem II efficiency and quantum yield for CO2 assimilation is not affected by nitrogen content in apple leaves.”
Adams WW III, Demmig-Adams B., Vernhoeven AS., and Barker DH., (1995) Photoinhibition during winter stress – Involvement of sustained xanthophyll cycle-dependent energy-dissipation. Aust J. Plant Physiol 22: 261-276 Journal of Experimental Botany, 55(403):1607-1621
Journal of Experimental Botany, 52(362):1865-1872Crafts-Brandner S. J., Law R.D. (2000) Effects of heat stress on the inhibition and recovery of ribulase-1, 5- biphsphate carboxylase/ oxygenase activation state. Planta (2000) 212: 67-74
all’Osto L, Cazzaniga S, Wada M, Bassi R. (2014) On the origin of a slowly reversible fluorescence decay component in the Arabidopsis npq4 mutant. Phil. Trans. R. Soc. B 369: 20130221.http://dx.doi.org/10.1098/rstb.2013.0221
da Silva J. A. & Arrabaca M.C. (2008).Physiologia Plantarum Volume 121 Issue 3, Pages 409 – 420 2008
Eichelman H., Oja V., Rasulov B., Padu E., Bichele I., Pettai H., Niinemets O., Laisk A. (2004) Development of Leaf Photosynthetic Parameters in Betual pendula Roth Leaves: Correlation with Photosystem I Density, Plant Biology 6 (2004):307-318
Eyodogan F., Oz M. T. (2007) Effect of salinity on antioxidant responses of chickpea seedlings. Acta Physiol Plant (2007) 29:485-493
Flexas 1999 – “Water stress induces different levels of photosynthesis and electron transport rate regulation in grapevines”J. FLEXAS, J. M. ESCALONA & H. MEDRANO Plant, Cell & Environment Volume 22 Issue 1 Page 39-48, January 1999
Flexas 2000 – “Steady-State and Maximum Chlorophyll Fluorescence Responses to Water Stress In Grape Vine Leaves: A New Remote Sensing System”, J. Flexas, MJ Briantais, Z Cerovic, H Medrano, I Moya, Remote Sensing Environment 73:283-270 Physiologia Plantarum, Volume 114, Number 2, February 2002 , pp. 231-240(10)
Gonias E. D. Oosterhuis D.M., Bibi A.C. & Brown R.S. (2003) YIELD, GROWTH AND PHYSIOLOGY OF TRIMAX TM TREATED COTTON, Summaries of Arkansas Cotton Research 2003
Hendrickson L., Furbank R., & Chow (2004) A simple alternative approach to assessing the fate of absorbed Light energy using chlorophyll fluorescence. Photosynthesis Research 82: 73-81
Kramer D. M., Johnson G., Kiirats O., Edwards G. (2004) New fluorescence parameters for determination of QA redox state and excitation energy fluxes. Photosynthesis Research 79: 209-218
Krause G.H., Weis E. (1984) Chlorophyll fluorescence as a tool in plant physiology. II. Interpretation of fluorescence signals. 5, 139-157.
Krupa Z., Oquist G., and Huner N., (1993) The effects of cadmium on photosynthesis of Phaseolus vulgaris – a fluorescence analysis. Physiol Plant 88, 626-630
D Edwards GE and Baker NR (1993) Can CO2 assimilation in maize leaves be predicted accurately from chlorophyll fluorescence analysis? Photosynth Res 37: 89–102
Laisk A and Loreto F (1996) Determining photosynthetic parameters from leaf CO2 exchange and chlorophyll fluorescence. Ribulose-1,5-bisphosphate carboxylase / oxygenase specificity factor, dark respiration in the light, excitation distribution between photosystems, alternative electron transport rate, and mesophyll diffusion resistance. Plant Physiol 110: 903–912
Photosynthesis in the water-stressed C grass is mainly limited by stomata with both rapidly and slowly imposed water deficits. Flexas (2002) Steady-state chlorophyll fluorescence (Fs) measurements as a tool to follow variations of net CO2 assimilation and stomatal conductance during water-stress in C plants Flexas J., Escalona J. M., Evain S., Gulías J., Moya I., Charles Barry Osmond C.B., and Medrano H. 4 Setaria sphacelata
Earl H., Said Ennahli S., (2004) Estimating photosynthetic electron transport via chlorophyll fluorometry without Photosystem II light saturation. Photosynthesis Research 82: 177186, 2004.Laisk A., Oja V, Eichelmanna H., Luca Dall'Osto L. (2014) Action spectra of photosystems II and I and quantum yield of photosynthesis in leaves in State 1, Biochimica et Biophysica Acta 1837 (2014) 315–325
Loriaux S.D., R.A Burns,Welles J.M., McDermitt D.K. Genty B. (2006) “Determination of Maximal Chlorophyll Fluorescence Using A Multiphase Single Flash of Sub-Saturating Intensity”. Abstract # P13011 August 1996.
American Society of Plant Biologists Annual Meetings, Boston MA LORIAUX S.D, AVENSON T.J., WELLES J.M., MCDERMITT D.K., ECKLES R. D., RIENSCHE B. & GENTY B. (2013) Closing in on maximum yield of chlorophyll fluorescence using a single multiphase flash of sub-saturating intensity Plant, Cell and Environment (2013) 36, 1755–1770 doi: 10.1111/pce.12115
Maai E., Shimada S., Yamada M.,, Sugiyama T., Miyake H., and Taniguchi M., (2011) The avoidance and aggregative movements of mesophyll chloroplasts in C4 monocots in response to blue light and abscisic acid Journal of Experimental Botany, Vol. 62, No. 9, pp. 3213–3221, 2011, doi:10.1093/jxb/err008 Advance Access publication 21 February, 2011
Moradi F. and Ismail A. (2007) Responses of Photosynthesis, Chlorophyll Fluorescence and ROS-Scavenging Systems to Salt Stress During Seedling and Reproductive Stages in Rice Annals of Botany 99(6):1161-1173
Nedbal L. Whitmarsh J. (2004) Chlorophyll Fluorescence Imaging of Leaves and Fruits From Chapter 14, “Chlorophyll a Fluorescence a Signature of Photosynthesis”, edited by George Papaqeorgiou and Govindjee, published by Springer 2004, PO Box 17, 3300 AA Dordrecht, TheNetherlands, page 389 -407
Netondo G., Onyango J., and Beck E., (2004) Sorghum and Salinity I. Response of Growth,Water Relations, and Ion Accumulation to NaCl Salinity, Crop Science 44:797-805
Siffel P., & Braunova Z., (1999) Release and aggregation of the light-harvesting complex in intact leaves subjected to strong CO2 deficit. Photosynthesis Research 61: 217-226
Strasser R.J, Tsimilli-Michael M., and Srivastava A. (2004) - Analysis of Chlorophyll a Fluorescence Transient. From Chapter 12, “Chlorophyll a Fluorescence a Signature of Photosynthesis”, edited by George Papaqeorgiou and Govindjee, published by Springer 2004, PO Box 17, 3300 AA Dordrecht, The Netherlands, page 340 Tripathy BC, Bhatia B., Mohanty P., (1981) Inactivation of chloroplast photosynthetic electron transport activity by Ni ++. Biochim Biophys Acta 638:217-224
Vredenberg W., Kay J. and Russotti R. (2013) The instrumental implementation of a routine for quantitative analysis of photochemical-induced variable chlorophyll fluorescence in leaves: Properties and prospects. ISPR conference in St. Louis, Poster e-mail: wim.vredenberg@wur.nl e-mail: Živ ák M., Bresti M., Olšovská K., Slamka P.(2008) Performance index as a sensitive indicator of water stress in PLANT SOIL ENVIRON., , 2008 (4): 133–139
Oquist G., and Huner N., (1991) Effects of Cold acclimation on the susceptibility of photosynthesis to photoinhibition in Scots pine and in winter and spring serials: A fluorescence analysis. Functional Ecology 5: 91-100